With three COVID-19 vaccines now available to the public, GW’s medical experts said the public should accept any of the vaccines first made available to them.
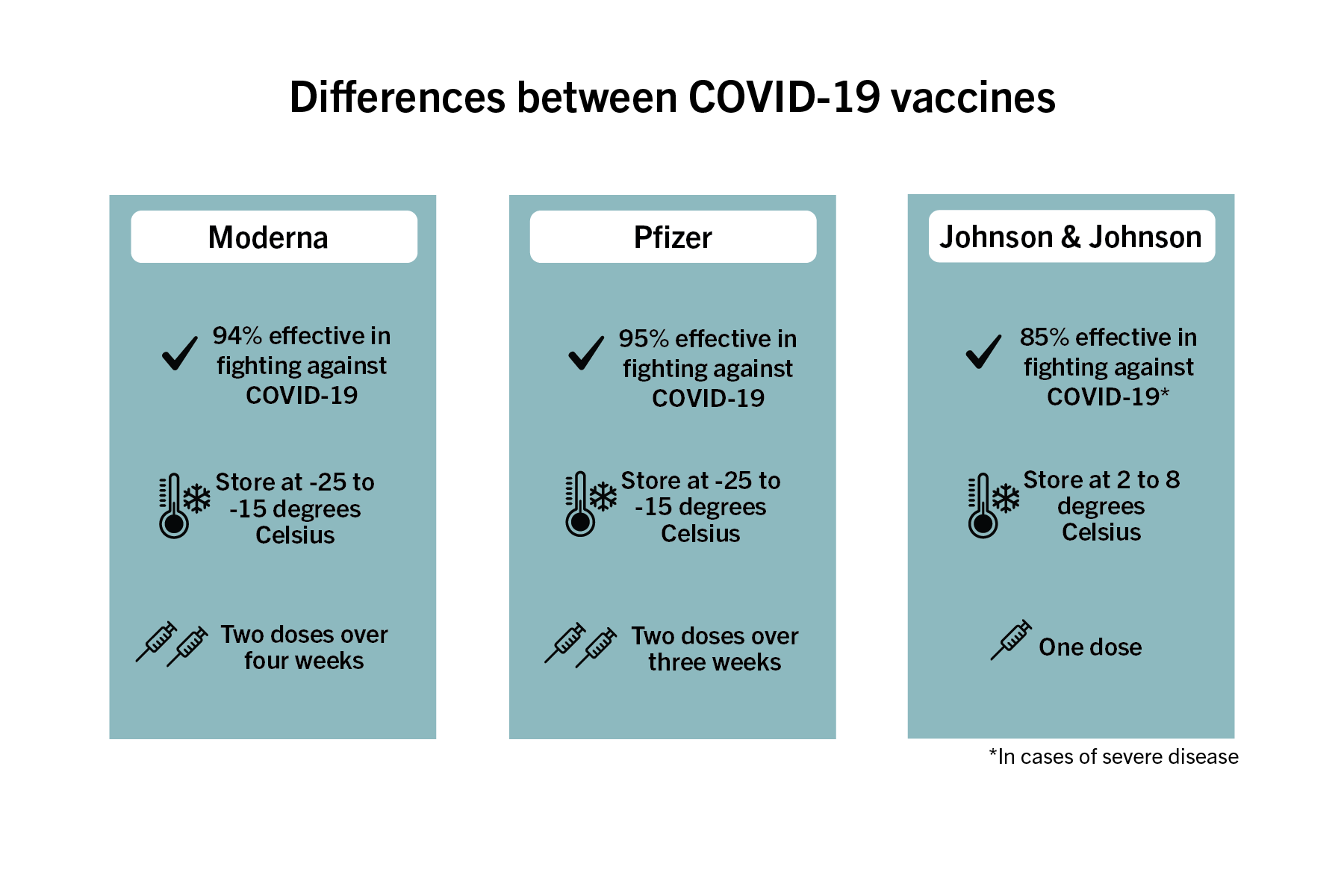
The Johnson & Johnson vaccine, which the Food and Drug Administration approved for emergency use late last month, was found to be 85 percent effective against the most severe COVID-19 illnesses and only requires one dose rather than the two needed for both the Moderna and Pfizer vaccines. Lynn Goldman, the dean of the Milken Institute School of Public Health, said the three vaccines are much more alike than they are different, and people should not prefer taking one vaccine over another.
“This is not a time to be shopping around for the best vaccines,” she said. “If the first one that’s available to you, they’re all effective. They’re all great vaccines.”
Sidney Lee | Graphics Editor
The Pfizer and Moderna vaccines are both more than 90 percent effective at preventing any illness from the virus. While all three vaccines were designed to fight against the original variant of the coronavirus, researchers have said the new variants, like those originating in the United Kingdom, South Africa and Brazil, are similar enough that the vaccine will likely still be effective in fighting off variants.
Despite having a lower efficacy rate than the Moderna and Pfizer vaccines, the Johnson & Johnson version was tested against some variants that were spreading at the time. The Pfizer and Moderna vaccines were only tested while the original variant of the virus was present, but a third booster shot to increase efficacy against the variants is being studied.
Goldman said both the Pfizer and Moderna vaccines inject RNA encased in a lipid-packaging agent, which commands the cells in the body to create proteins that can trigger an attack on the coronavirus when it enters the body. She added that the mRNA used in the vaccines tends to degrade easily, requiring that the vaccine be kept at extremely low temperatures.
“RNA is one of the trickiest things to handle, so they have packaged it up inside these little lipid balls and then they have to freeze it,” Goldman said. “That’s why they have to keep it cold or it will degrade.”
The Pfizer vaccine originally needed to be stored at temperatures as cold as -80 degrees Celsius but now can be stored at between -25 and -15 degrees. The Moderna vaccine must be stored at about -20 degrees Celsius while the Johnson & Johnson vaccine can be stored at between 2 and 8 degrees Celsius.
Goldman said the Johnson & Johnson vaccine uses an inactivated adenovirus, which is not harmful to humans, to package DNA rather than the synthetic RNA found in the Moderna and Pfizer vaccines. The DNA codes the cell to produce RNA, which creates the same proteins that can be produced by the Pfizer and Moderna vaccines.
“That is an extra step in the process, but it is not a slow step and it doesn’t really change anything,” she said.
Goldman said the goal of all three of the vaccines is to recognize the proteins found on the outside spikes of a coronavirus and create a targeted immune response toward the part of the virus that makes it infective.
She said comparing the effectiveness of the three vaccines is difficult because each clinical trial was slightly different in terms of design and the selected populations. Goldman said Johnson & Johnson’s could potentially turn out to be overall more effective because people can get vaccinated with a single shot compared to Pfizer and Moderna vaccines, which require people get a second shot a few weeks after the first.
“On the other hand, you have to have two shots to get those, and some percentage of the population will never show up for that second shot,” she said. “So from the standpoint of public health, it could be that at the end of the day, the J and J will be more effective for the population.”
Nirbhay Kumar, a professor of global health in Milken, said the side effects of the Moderna and Pfizer vaccines could include fever, body aches and muscle aches. The side effects of the Johnson & Johnson vaccine could include the same symptoms, according to a Johnson & Johnson release.
Kumar said these potential side effects should be seen as a “good thing” because it means the vaccine is effective in fighting the virus.
“If it is doing its job then that means there is a good likelihood of a good possibility that you are going to have effective immunity that is going to provide the protection you need,” he said.
Kumar said the adenovirus in the Johnson & Johnson vaccine lasts longer in the body and could potentially be just as effective as the Moderna and Pfizer vaccines, which require two doses.
“The adenovirus that is in the J & J vaccine the vaccine itself can last longer in the body,” he said. “It means it is more like getting infected and the infection is giving you better immune responses, so I think the single dose is equally effective for the J & J vaccine as compared to the Moderna and Pfizer vaccine.”
Neil Johnson, a professor of physics in the Columbian College of Arts and Sciences, said he has researched the online arguments between pro- and anti-vaccination views since the pandemic began last year. He said he has found more of a brand hesitancy than a vaccine hesitancy in his research that is continuing as new companies unveil their COVID-19 vaccines.
He said he’s noticed that people are now starting to discuss the pros and cons of each vaccine, instead of having equal trust in all of them. He added that he’s concerned that a drop in the trust in one of the vaccines could set back the push for herd immunity.
President Joe Biden unveiled a plan last week that would expedite the production of COVID-19 vaccines, resulting in enough vaccines for all Americans by the end of May. But experts warn the distribution of those vaccines could take much longer.
“What we see is instead of it being the vaccine, we begin to see a lot of discussion about particular types,” Johnson said. “It is almost like the discussion of, ‘OK, you are going to get a smartphone. Everyone should have a smartphone.’ Then it comes down to, ‘OK, which smartphone are you going to get? Which version?'”






